The European Commission has published an updated factsheet for authorities in non-EU/EEA states on medical devices and in vitro diagnostic medical devices. This factsheet seeks to further inform third country authorities on the changes and timelines concerning the Medical Devices Regulation 2017/745 (MDR) and In Vitro Diagnostic Devices Regulation 2017/746 (IVDR) in order to avoid market disruptions. Thus, it addresses relevant points such as:
- Conformity assessment and CE marking requirements
- Supply chain traceability and unique device identifiers (UDIs)
- Clinical evaluation requirements
- Post-market surveillance
- European Database on Medical Devices (EUDAMED)
This update includes the extended transitional period for legacy devices, as mentioned in our previous post. Moreover, it provides a clear infographic on the existing timelines under MDR:
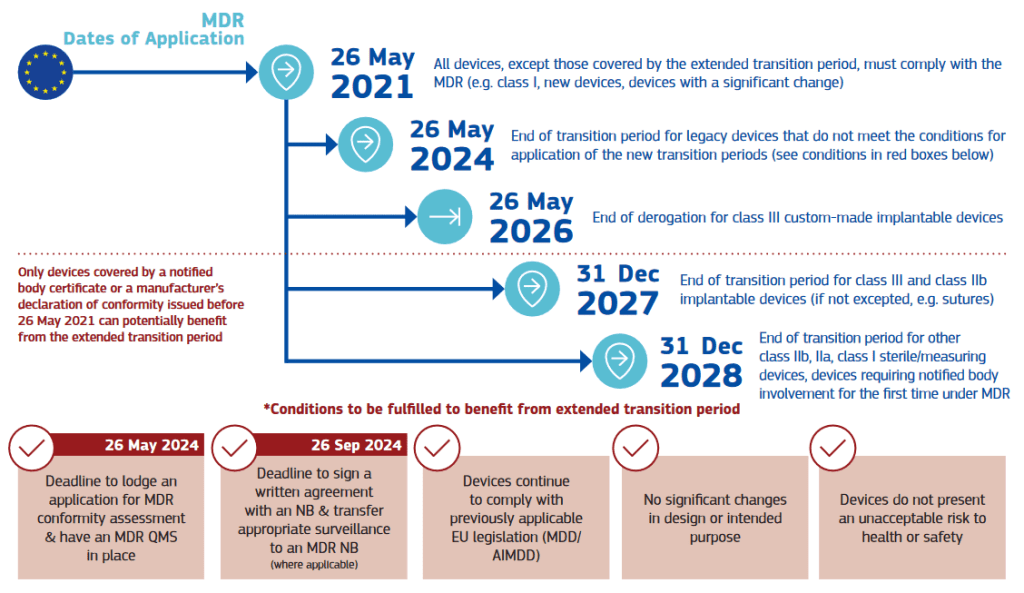
And under IVDR:

Source of images: European Commission
The factsheet also includes a section on Certificates of Free Sale, indicating that the existence of an MDR/IVDR Certificate does not automatically lead to the invalidation of the MDD/AIMDD or IVDD Certificate under which the Certificate of Free Sales has been issued. As per legacy requirements, valid MDD/AIMDD or IVDD certificates remain valid until the corresponding deadline stated above.
You can read more about this in our MDR & IVDR European Commission section!


Leave a Reply