On 10 July 2025, the Medical Device Coordination Group (MDCG) released a position paper to clarify the timelines of the implementation of the Master UDI-DI for contact lenses and spectacle frames, spectacle lenses, and ready-to-wear reading spectacles on the Union market. The Master UDI-DI is a unique identifier for highly individualised medical devices that share specific clinically relevant parameters. This common identifier would be used in the UDI/Device registration module of EUDAMED. Thus, manufacturers, distributors, and the EUDAMED database will not have too many identifiers assigned for similar devices for the EU market.
Reference dates for Master UDI-DI
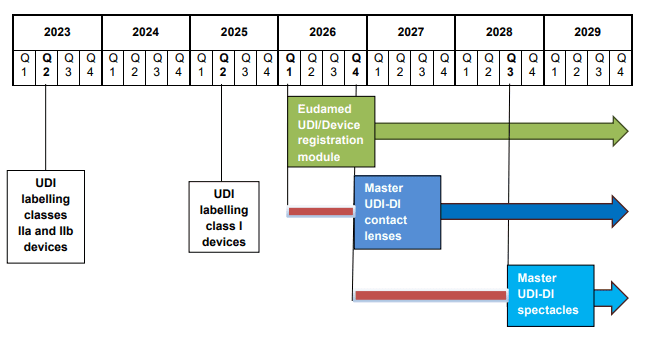
The MDCG Paper highly encourages manufacturers of contact lenses, spectacle frames, spectacle lenses, and ready-to-wear reading spectacles to make use of the voluntary assignment of Master UDI-DI before the mandatory dates of application.
How to assign a Master UDI-DI
The assignment of a Master UDI-DI follows EU-designated issuing entity rules and must be linked to a Basic UDI-DI. The issuing entities are GS1, HIBCC, ICCBBA, and IFA.
Do you have questions on UDI obligations? Contact us here or at mdlaw@obelis.net.


Leave a Reply